Researchers from Kyoto University’s Graduate School of Medicine and Kyoto University Hospital (Professor Miki Nagao, Associate Professor Yasufumi Matsumura, Lecturer Masaki Yamamoto and Assistant Professor Taro Noguchi) has developed a method that enables high-throughput screening for SARS-CoV-2 by employing fully automated genetic testing systems and demonstrated the usefulness of this method.
There has been a desire for reliable mass diagnostic testing methods as a control measure for the COVID-19 epidemic. Professor Nagao’s group aimed to develop a highly accurate mass sample screening system by combining a sample pooling method and commercially available fully automated genetic testing devices. In this project, three automated genetic testing systems were evaluated: the geneLEAD system (Precision System Science Co., Ltd.), the Panther system (Hologic Japan Co., Ltd.), and the Biomek system (Beckman Coulter Co., Ltd.). The group developed and optimized systems that combined each of these devices with automated dispensers manufactured by Precision System Science Co., Ltd., Beckman Coulter Co., Ltd., or Integra Biosciences Co., Ltd. and evaluated the performance of each system by testing several thousand clinical samples.
The outcome of this study is expected to facilitate the introduction of high-precision mass testing systems and contribute to the control of the spread of COVID-19.
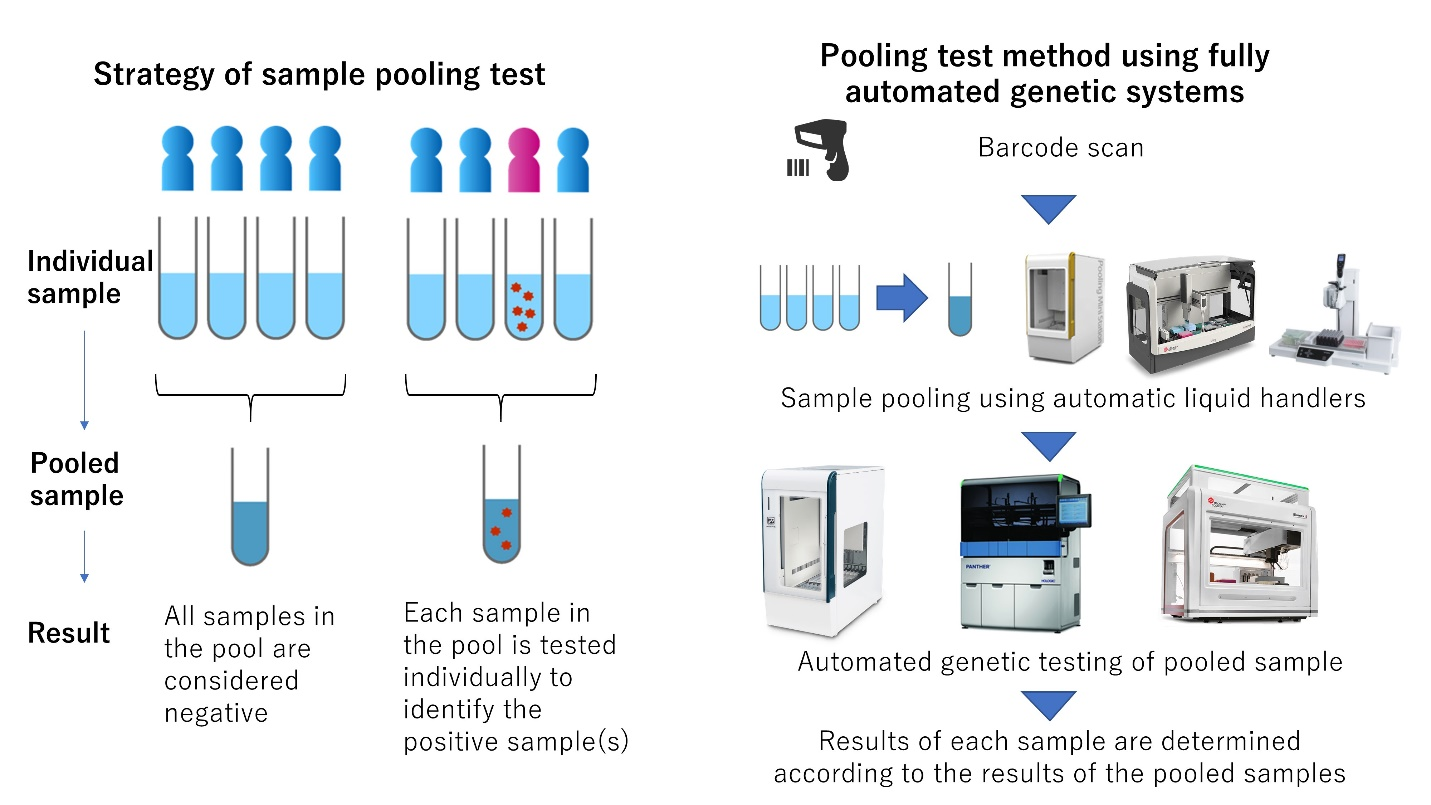
Left: Strategy of sample pooling test / Right: Pooling test method using fully automated genetic systems
SARS-CoV-2, the causative agent of COVID-19, can be transmitted not only by individuals with clinical symptoms but also by asymptomatic carriers. Establishing a rapid, accurate, and cost-effective mass screening scheme for routine screening of individuals at high risk of infection (essential workers and cross-border returnees, for example) or identifying infected individuals within cluster cases could contribute to achieving a balance between meeting public health demands and maintaining socioeconomic activity during the COVID-19 pandemic.
The "pooled" testing method, in which multiple samples are pooled together as a single sample, theoretically increases the number of tests that can be performed simultaneously and thus contributes to the reduction of testing costs. For example, pooling groups of 6 samples from a population with a positivity rate of 1% could reduce the number and cost of tests to approximately one-fifth of the cost of screening all samples. In an actual test procedure, if a pooled sample tests positive for SARS-CoV-2, each sample in that pool is then tested individually to identify the positive sample(s) (Figure). If the pooled sample tests negative, all samples in that pool can be considered negative for SARS-CoV-2, and no further tests are necessary.
There are some concerns with the pooled testing strategy, including lower performance due to dilution of positive samples by negative samples in the pool and compromised accuracy due to cross-contamination. As genetic testing is technically demanding, automation of the testing process is beneficial to avoid errors and to achieve high accuracy. However, no existing automated testing system is equipped with the ability to perform sample pooling. Therefore, here, multiple sample pooling test systems were developed by combining a highly accurate nucleic acid amplification test method and automated testing systems (Figure), and their effectiveness was evaluated by testing a large number of patient samples.
The sample pooling test systems were developed utilizing three different automated devices capable of high-precision mass testing: (1) geneLEAD VIII and geneLEAD 24 (Precision System Science Co., Ltd.), the Panther system (Hologic Japan Co., Ltd.), and (3) the Biomek system (Beckman Coulter Co., Ltd.). The developed systems were evaluated for basic performance, and then a clinical validation study was conducted by testing over 2000 patient samples. The performances of the developed systems were compared to the reference method published by the National Institute of Infectious Diseases (NIID).
(1) The geneLEAD system can simultaneously process up to 8 samples (geneLEAD VIII: commercially available) or 24 samples (geneLEAD 24: in preparation for commercial launch) in approximately 2 hours. Pooled sample tests on the geneLEAD system are enabled by employing a "Pooling Station" (Precision System Science) that is capable of quick sample barcode reading and pooling. Pooling of up to 8 samples is possible with the Pooling Station, but 6-sample pooling was found to be optimal for the subsequent analysis on geneLEAD VIII, meaning that 48 samples per batch can be processed in approximately 2.5 hours. The optimal pooling size was 8 when geneLEAD 24 was used for analysis, meaning that up to 192 samples per batch could be tested in approximately 3.5 hours. In the validation study, a total of 2448 saliva and swab samples were tested, and the results showed a sensitivity of 97.1% and a specificity of 99.9%, demonstrating the high accuracy of this system.
(2) The Panther system allows random-access sample loading and simultaneous sample processing. Analysis can be performed in 3.5 hours for a single sample or 5.5 hours when the maximum of 120 samples is loaded. The research group developed a sample management system using the Biomek system (Beckman Coulter Co., Ltd.) and the ASSIST PLUS system (Integra Biosciences Co., Ltd.) in cooperation with the manufacturers of these instruments. The pooling test method using the Panther system in combination with this sample management system enables automated pooling tests of up to 10 samples at a time. The optimal pooling size was determined to be 4 based on the basic performance evaluation, and 384 samples could be processed in approximately 5 hours. In the validation study, a total of 3228 nasal swab samples were tested, and the results showed a sensitivity of 95.6% and a specificity of 99.9%, demonstrating the high accuracy of this system.
(3) The Biomek system is a general-purpose device for research, and it can process 94 samples simultaneously in approximately 2.5 hours by incorporating a real-time PCR instrument and using the NIID assay. In collaboration with Beckman Coulter Co., Ltd., the research group developed a program for barcode management, sample pooling, and the subsequent analysis of pooled samples using the Biomek system. This program enables automatic testing of any number of samples. The basic performance test indicated that the optimal pool size was 4, and 376 samples could be processed in approximately 4 hours. In the validation study, a total of 6420 saliva and swab samples were tested, and the results showed a sensitivity of 97.9% and a specificity of 99.8%, demonstrating the high accuracy of this system.
The sample pooling systems described here use existing fully automated genetic testing systems. By applying the expertise necessary for sample pooling provided by Kyoto University and the manufacturers of these systems, these systems can be readily introduced to other testing facilities. They would enhance the capacity to perform mass sample testing with high accuracy and can be expected to contribute to controlling the spread of COVID-19 in Japan.
The grants and collaborations associated with this project are as follows:
・ Japan Agency for Medical Research and Development (AMED) 2nd year of Reiwa virus and other infectious disease control technology development project (support for empirical research toward the realization of early and mass infectious disease testing) “Clinical validation study of safe, large-scale, accurate and fully automated molecular testing systems for COVID-19"
・ Joint research with the Center for iPS Cell Research and Application, Kyoto University, “Establishment of SARS-CoV-2 molecular and antibody testing systems, enhancement of diagnostic test capacity in clinical laboratories, and epidemiological study of COVID-19”
・ Joint research with Precision System Science Co., Ltd. “Evaluation research of SARS-CoV-2 test PCR reagent using fully automated gene test device geneLEAD series and related systems”
・ Joint research with Beckman Coulter Co., Ltd. “Development of a sample pooling method using automated liquid handling w Biomek 4000”
・ Joint research with Integra Biosciences Co., Ltd. "Development and validation of diagnostic test methods for COVID-19 using an automated dispenser”
Nucleic acid amplification test: One of the methods available to determine the presence or absence of a pathogen by amplifying a gene (nucleic acid) unique to the pathogen. With the appropriate reagents, extremely accurate testing is possible. In addition to the PCR method used by GeneLEAD, there are several types, such as the TMA method used by the Panther system.
geneLEAD: A fully automated general-purpose genetic testing system manufactured and sold by Precision System Science Co., Ltd. that can also be used for the diagnosis of COVID-19. Eight to 24 samples can be processed at the same time.
Panther system: A fully automated genetic testing system sold by Hologic Japan Co., Ltd. that enables easy mass testing when using a specific reagent and sampling kit. The system can be used for the diagnosis of COVID-19.
Biomek system: A fully automated general-purpose liquid dispenser sold by Beckman Coulter Co., Ltd. Biomek 4000 was used for sample pooling, and Biomek i5 was used for fully automated genetic testing. Although it is a device for research use, sample pooling and nucleic acid amplification tests for the diagnosis of COVID-19 can be automated by developing a program for the Biomek system and combining it with the reference PCR method established by the NIID.
Sensitivity and specificity: An index demonstrating the test performance. Sensitivity is the proportion of true positive samples that test positive, and specificity is the proportion of true negative samples that test negative.